In addition to carefully monitoring the nutrients and EC fed to the plants, we also pay a lot of attention to the pH of the feed water. Why do we do this? Why is the pH so important, and what is its influence on the plant?
The pH value represents the negative logarithm of the concentration of hydrogen ions in a solution. The pH is therefore a measure of acidity. Raising or lowering the pH by one point means that the solution becomes ten times more alkaline or acidic. Each point is therefore factor ten in concentration. This means that a pH of four is 1000 times more acidic than a pH of seven! This is why pH plays such an important role in our crops. Water that is too acidic has a negative impact on the plant and its root environment. This is what we will be discussing further in this article.
pH watering
A certain pH is standard in the substrate of the plants. For a good root system, it is important that the pH of the substrate and the root are not too far apart. When watering, you want to change the pH value of the substrate and the root as little as possible. By keeping this stable, the plant will be able the best to absorb nutrients.
Nutrient uptake by the plant
The pH has an important influence on the nutrient uptake of the root. If the pH is too high or too low, nutrients cannot be absorbed optimally. For plants, the ideal pH of a nutrient solution is between five and six. When the pH is higher than six, plants can have problems with the absorption of boron, copper and phosphates. Substances also precipitate earlier when the pH is high; this can be done in the fertilizer tank as well as in the pipe network.
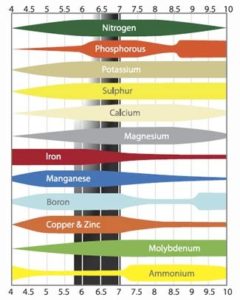
When the pH becomes too low – i.e., below five – the plant will find it difficult to absorb other substances. The main problem with too low a pH is the absorption of molybdenum. At a low pH it also becomes more difficult for the plant to absorb nitrogen and sulphur. If the pH is really low, poisoning can occur. Plants easily absorb manganese and aluminium at a low pH, which can poison the plant. A brief overview of the information given above can be found in the figure below.
| Hard or impossible to absorb at pH ≤5 | Hard or impossible to absorb at pH ≥6 |
| Nitrogen (N) | Boron (B) |
| Molybdenum (Mo) | Phosphates (PO) |
| Sulphur (S) | Copper (Cu) |
Although the pH of the irrigation water is usually monitored, there can be a difference with the pH of the substrate or drain. The accumulation of certain elements that are not absorbed by the plants or excreted by them usually causes the pH to drop. This relates to the composition of the substrate. Each substrate has different properties with regard to the moisture balance, which influence the pH. A substrate that drains minimally builds up EC, which often means that the pH will drop.
This figure shows at which pH the elements are best absorbed by the plants.
pH in the fertilizer tanks
To dissolve the fertilizers in the fertilizer tank, it is necessary that the pH does not exceed six. Our advice is to maintain a pH of between four and five so everything will dissolve properly. It is also important that iron does not go into the same container as phosphoric and nitric acid. The acids corrode the iron chelates with the result that the iron oxidizes and the absorbable Fe3+ will then connect to O2 and change into non-absorbable Fe2O3 .
Monitoring
When measuring the pH, it is important to do this as soon as possible after taking the sample: the pH of a water sample changes over time. It is important to take fresh water for a sample and not old, stagnant water. For these reasons, it is of little or no use to send samples to laboratories for pH measurement.
Clean the pH measuring instrument regularly and calibrate it several times a year. Over time, it is possible that the measuring equipment will indicate incorrect values, which can result in water with an incorrect pH going to the plants.
Hand-held measuring instrument
The sensor of a pH measure instrument is very sensitive so it is better not to touch it with bare hands. The naked hand can cause damage, resulting in incorrect measurements. The sensor should be cleaned after each measurement. It is best to rinse it with demineralized water so that no harmful substances remain. It is also important to store the sensor in a moist environment so that no dry residue accumulates on the sensor. To do this, put special storage liquid in the sensor cap.

Fixed measuring instrument
When working with an injection unit in which the fertilizers from the tanks are only mixed when they enter the greenhouse, it is important that the pH measurer is not too close to the mixing device. It should be two to three metres away from the mixing device because the pH is not yet stabilized here.
When there is dirt on the sensor, this can be removed by cleaning it with a soft cloth, soaking it in light soapy water for 15 minutes, or soaking it in alcohol.
Influence of the plant on pH
The plant itself has a strong influence on the pH because it selectively absorbs certain nutrients. If cations (positively charged ions) and anions (negatively charged ions) are absorbed equally, the pH in the substrate will remain the same. The electrical charge in the plant must remain neutral; this means that if the plant takes up potassium (K+), for example, it will release hydrogen (H+). Conversely, this means that if the plant absorbs nitrate (NO3–), for example, it will release hydroxide (OH–). When the plant absorbs as many cations as anions, the H+ will bind to the OH– and this will become the neutral water (H2O). If there is no balance between the two, the pH will change. As more hydrogen (H+) ions are released, the pH will drop, and as more hydroxide (OH–)-ions are released, the pH will rise.
Because the roots breathe, they also produce carbon dioxide (CO2). As a result, CO2 will also react with the OH– and hydrogen carbonate (HCO3) will be created. Plants absorb more cations than anions, which causes the pH to drop.
The moment a plant becomes generative, it absorbs more potassium (K+) and the pH will drop.
Nitrogen (N) in the form of ammonium (NH4+) is converted into nitrate (NO3–). This causes the hydrogen (H+) concentration in the substrate to increase and results in a lower pH. It is therefore important that when a lot of ammonium is administered, the pH of the input is high enough. This means that it must be above 5.5 in summer and slightly lower in winter, when less nitrogen is used by the plants. It is good to reduce this with ammonium.
Buffering the substrate
The pH can be buffered in the substrate by, for example, mixing Dolokal (CaCO3 + MgO) into it. When Dolokal is added to the substrate, it will slowly dissolve. As Dolokal dissolves, elements are released. These elements influence the pH, which will rise as a result. Normally Dolokal contains 5% magnesium oxide (MgO), but there is also Dolokal Extra that contains 10% MgO. The reaction in the soil of the carbonate group of Dolokal is as follows:
CO32- à H+ + CO32- à HCO3–
HCO3– à H+ + HCO3– à H2O + CO2
Bicarbonate (HCO3) is an element that does not add much in terms of nutrition to the plants, but it does have an important function for the pH in the substrate. If there is more HCO3 in the substrate the pH will be higher. In clay like substrates it is harder to flush out the HCO3, in more open substrates like bark it is easier to flush out.
The buffering of bicarbonate looks like this in a formula:
H2O + H+ à H3O + HCO3 à H2CO3 + H2O à 2H2O + CO2
Here you can see that the bicarbonate binds with H3O and that it eventually will be converted to water (H2O) and carbon dioxide (CO2).
Feed water is often buffered with potassium and this can be done in two forms: potassium bicarbonate (K2CO3) and caustic potash (KOH). Potassium bicarbonate has a slow action, so the effect will not be directly measurable. Caustic potash has a direct pH increasing effect.
Phosphate also has a buffering effect and can be used in different compositions. The phosphate compounds that buffer are the following:
PO43−, HPO42−, H2PO4− and H3PO4
Here you can see that phosphate has a triple buffering effect.
Remarks
The solubility of iron depends on the type of chelate administered. There are three different chelates that are stable at different pH values. The least stable chelate is EDTA and the most stable is EDDHA. Below you will find a diagram with the pH values in which the chelates are stable.
| Type of chelate | Stable at pH |
| EDTA | 3 – 6 |
| DTPA | 3 – 6.5 |
| EDDHA | 3 – 10 |
If the iron chelate is not stable, it will disintegrate and the iron will not remain dissolved. This ensures that iron goes from Fe2+ (dissolved) to Fe3+ (rust). This makes it important to choose the right type of chelate. When the iron comes loose from the chelate, it will immediately form a connection, converting into precipitated iron, which is no longer absorbable by the plants. The stronger the compound, the more expensive the iron becomes, because the production process for these chelates becomes more expensive.
pH in Phalaenopsis
In the case of Phalaenopsis, it is important to know that the pH in the pot will drop in time of cultivation. During the cooling phase of branch induction and the rest of the growth of the branch, the Phalaenopsis will absorb more potassium, causing the pH in the substrate to drop.
In winter, Phalaenopsis uses less nitrogen then in summer. This means that the pH may be slightly lower, because the plant receives enough nitrogen.

pH and cut Anthurium
Besides the influence of the cut Anthurium plant itself on the pH, acidity is mainly determined by the substrate in which it is grown. With stone wool, the pH remains high for a long time, while with perlite the pH in the drain can drop considerably after just six months of cultivation. The behaviour of the oasis is strongly dependent on the degree to which it is limed. Liming with Dolokal/Dolomite every year is often necessary for oasis-based cultivation. When growing on perlite, the pH in the drain can usually be kept at 3,0 by using the right pH and amount of bicarbonate (HCO3-) in the water.
The variety can have a major influence on the pH in the substrate or drain. Because of the selective uptake of elements, it can be observed that in light species (Moments®, Acropolis® and Angel®) the pH can quickly drop and reach very low levels. pH values of 2,0 are no exception. These low pH values make the absorption of Ca2+ very difficult, with the result that cell structures are not built up as easily. Problems with glaze or blue in the bracts can then easily occur.

Glazed flower caused by low pH
The pH level is very important for the plant, so always keep a close eye on the pH of the nutrient solution and what is fed to the plants. If you have any questions on this subject or on other any other technical cultivation issues, please contact Bureau IMAC Bleiswijk.







