In the Anthurium and Phalaenopsis cultivation, a lot of attention is paid to acidity. The acidity, or pH value, is a logarithmic scale indicating the number of H+ ions. Water with a neutral pH of 7 has as many H+ ions as OH– ions. A pH below 7 is acidic and a pH above 7 is basic (alkaline). The scale is logarithmic, which means that a pH of 6 is ten times more acidic than a pH of 7.
The pH value is important for the absorption of several nutritional elements. At a high or low pH, certain nutrients cannot be absorbed properly. For both Anthurium and Phalaenopsis, the optimal pH is between 5 and 6. At a high pH, substances such as boron, phosphates and copper are difficult to absorb. At a low pH, nitrogen, molybdenum and sulphur are difficult to absorb. At an extremely low pH, symptoms of poisoning can also occur due to manganese or aluminium, for example.
| Hard or impossible to absorb at pH <5 | Hard or impossible to absorb at pH >6 |
| Nitrogen (N) | Boron (B) |
| Molybdenum (Mo) | Phosphates (P) |
| Sulphur (S) | Copper (Cu) |
Irrigation water
When rain or osmosis water is used, there are very few dissolved substances in the water. Irrigation and tap water naturally contains bicarbonate, which has a pH buffering effect. Rainwater does have a pH of about 6.5, but does not contain a buffer, so it can change its pH quickly. It is therefore advisable to add bicarbonate to the water. A good dose is 36g/m3; this gives about 0.35 mmol HCO3–.
Depending on the pH of the starting water and possibly recirculation water, the irrigation water is either acidified or leached by pH control. It is important to include sufficient acid or alkali in the fertilization schedule. If the pH control still needs a lot of adjustment, it is possible that not all the acid or alkali has worked itself out before it reaches the pH sensor of the pH control. Because of this, too much acid or alkali can be added.
It is therefore necessary to measure the irrigation water from the pipeline at least once a month. You should do this after the pipe has been completely refreshed so there is no old stagnant water remaining from the previous irrigation session. This is especially important if you don’t work with a day’s supply. With a day’s supply, the irrigation water has been standing for some time and the fertilizers have had time to react. Usually the pH of the day’s supply is also brought up to the required level by means of an acid/alkali regulation before it enters the greenhouse.
Fertilization tanks
To dissolve the fertilizers in the tank, it is important that the pH does not exceed 6. Our advice is to maintain a pH of between 4 and 5 so everything will dissolve properly. It is also important that iron does not go into the same container as phosphoric and nitric acid. The acids corrode the iron chelates with the result that the iron oxidizes and the absorbable Fe3+ will bind to the O2 and change into non-absorbable Fe2O3 (rust).
Acidity of the substrate
The pH of the substrate is often different from that of the irrigation or drain water. This is caused by two processes: the uptake of nutrients by the plant and the conversion of fertilizers by soil organisms.
Of these processes, the uptake of nutrients by the plant has the greatest influence. The electrical charge in the plant must remain neutral; this means that if the plant takes up potassium (K+), for example, it will release hydrogen (H+). On the other hand, it also means that if the plant absorbs nitrate (NO3–), for example, it will release hydroxide (OH–). If cations (positively charged ions) and anions (negatively charged ions) are absorbed equally, the pH in the substrate will remain the same. In this case, the H+ excreted by the plant will bind with OH– and form water (H2O). If there is no balance between the H+ and the OH–, the pH will change.
Because the roots of the plants breathe, CO2 is produced in the substrate. When free OH– is present, it will react with the CO2, creating bicarbonate (HCO3–). In the event of an anion uptake, the pH of the substrate will increase.
Influence of soil organisms
Ammonium-containing fertilizers are converted to nitrate by soil organisms. This process is called nitrification. This goes according to the following reaction:
NH4 + 2 O2 -> NO3 + 2H+ + H2O
In this process H+ is released, which has an acidifying effect. When urea is used, it is first converted to ammonium by urease. This goes according to the following reaction:
![]()
This reaction causes a slight increase in pH because ammonia is alkaline. After this, however, nitrification of ammonia also takes place, again releasing H+. Net urea therefore also has an acidifying effect.
During generative growth, more potassium is absorbed than during vegetative growth. As the plant ages, more H+ is released and the pH decreases.
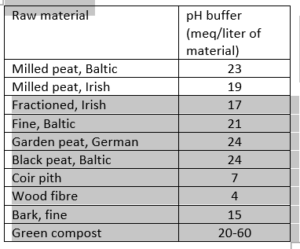
To counteract pH fluctuations, substrate is often buffered. However, there is a difference in the buffer capacity of substrates; peat, for example, has a larger buffer capacity than coco peat. The buffer size is indicated in meq or mmol per litre. Substrates with a small pH buffer are less able to absorb fluctuations and therefore react more unpredictably. The buffer can also wear off over time, usually after 8-12 weeks.
pH and Phalaenopsis
Measures
In Phalaenopsis, the acidification of the substrate is a natural process; during the induction and finishing phases, the pH can decrease significantly. Fluctuations in the pH, for example during irrigation, may cause black root tips.

Poor root quality in Phalaenopsis.
These damaged roots are points where fusarium and pythium can penetrate the plant. That is why it is important to absorb pH fluctuations in the substrate. There are several possible measures:
Adding lime to the substrate
At the start of the cultivation period you can add about 3kg/m3 of Dolokal. Dolokal contains calcium carbonate, magnesium carbonate and magnesium oxide. During cultivation, these substances are released from the powdered lime. In an acidic environment, the carbonate will absorb H+ ions, preventing a decrease in the pH. The disadvantage of Dolokal is that it washes away quite quickly in a coarse substrate such as bark. This is why it is especially active during the first few weeks.
Reducing ammonium and urea
As described earlier, the nitrification of ammonium releases H+, which has an acidifying effect. H+ is also excreted by the uptake of NH4– by the plant. This is why ammoniacal nitrogen is often replaced by nitrate-nitrogen when the substrate pH decreases. If liquid fertilizers are used, ammonium administration can be reduced to 0 mmol if necessary. When solid fertilizers such as calcium nitrate are used, there will always be some ammonium present; the lowest achievable level will be about 0.25 mmol of ammonium. Blended fertilizers such as PG-mix and Osmocote also contain a reasonable amount of ammonium.
Rinse with clean water
When the substrate is poorly flushed, fertilizers and root exudates accumulate. This is often associated with a decreasing pH. By rinsing with clean water with a pH of approximately 6.5, the substances in the substrate are diluted, and the pH can be restored.

Good root quality in Phalaenopsis.
pH and Anthurium
In general, Anthurium grow better at a lower pH than a higher one. Higher pHs (>6.2) quickly diminish leaf and flower shine due to poorer growth. In addition, high calcium (Ca++) and bicarbonate (HCO3–) administration can contaminate the crop by calcium carbonate (CaCO3) precipitation.

Leaf contamination from calcium carbonate (CaCO3).
The pH of Anthurium varies according to the season. In spring, the pH generally rises due to the increase in natural growth. The plant absorbs extra NO3– during this time, causing the pH to rise. In the autumn, on the other hand, more positively charged ions such as K+ and Ca++ are absorbed. This causes the pH to decrease. The use of ammonium-containing fertilizers can also have a significant pH-decreasing effect. This happens especially in winter, when more solid calcium nitrate is given. The ammonium (NH4+) input can then increase towards values of 0.5-0.7 mmol/l, which has a strong acidifying effect. In this case, it is best to switch to liquid calcium nitrate, since it does not contain NH4+.
During cultivation, the pH decreases and therefore the drain water in general has a low pH. If the irrigation water consists of a large percentage of drain water, additional pH correction may be necessary.
A low pH makes the flowers more sensitive to problems such as blue tones and glaze.

Blue in the bracts.
Pot Anthurium
With pot Anthurium we generally see few problems with fluctuating pH levels in the pot because the cultivation takes place on organic substrates and is relatively short. Peat substrates are limed (usually with Dolokal) to achieve a neutral (about 5.6-5.8) pH at the start of cultivation. However, the combination of the use of well water and alkaline substrate can lead to pH values that are too high. A reduction in the shine of the plant can be the result, because the absorption of trace elements is strongly hindered at higher pH levels. Therefore, you should aim for a pH of between 5.2-5.8 in the pot.
Cut Anthurium
Three factors influence the course of pH levels in a cut Anthurium crop:
-control of the variety
-substrate influences
-pH of irrigation water
Control of the variety
The specific variety can have a major influence on the pH in the substrate or drain water. Due to selective element uptake, in light species (Moments®, Acropolis® and Angel®) the pH can drop quickly and reach very low levels. pH values of 2.0 are no exception.

Low pH on pH meter.
Influence of the substrate
The substrate on which the plant grows has an enormous influence on the pH in the root medium and the drain water. With rock wool, the pH often remains high, while with perlite the pH in the drain water can drop considerably after 6-9 months of cultivation. Oasis is acidic in itself, but is immediately limed with Dolokal/Dolomite from the start.
Irrigation water
Growers who use spring water as the starting water tend not to have any problems with an excessively low pH. We generally see high pH problems in these cases.
pH in the root environment/drain water
The pH in the substrate is generally higher than in the drain water. This can differ by 1-2 pH points. With a drain water pH of 4, the pH in the root environment can still be 5 or higher.
Because of the long cultivation time of cut Anthurium, the pH in the root environment and drain water can become very low. Up to a pH of 3.0 in the drain water there is generally no cause for concern. A drain water pH below 3.0 is not desirable, because direct root damage can occur. This can be so serious that the roots die off completely within a few weeks. This is also called root reduction.

Root reduction due to very low pH in the root environment.







